Welcome to RK Research Group
The natural world is of course a highly complex system where only a very minimum understanding could be gathered so far and seems impossible to understand in a better way during the entire course of mankind, whatever science and technology emerges in future. However, it is highly important to understand at least a minimum so as to improve the quality of life, not only of the humans but also of the entire flora and fauna.
We believe that problems associated with various aspects of life could only be solved through fundamental research in it. For instance, understanding chemical reactions starts from understanding the characteristics of atomic or molecular orbitals, the manifestations of free energy using the so-called catalysts has a central role in the formation/breakage of bonds which are difficult to make or break otherwise. Such approaches would lead to accessibility of a variety of pharmaceutical intermediates/ bioactive molecules. Problems and challenges that we consider are like a do or die strategy where results are seldom, but experimentations and understandings would finally bring something marvellous, that’s the way of science. We are interested only in finding out the new that creates new knowledge in science and not interested in quantification or analysis of what is something already there around.
It is the curiosity and motivation that lead us to do science. We work on solving some of the fundamental problems associated within organic synthesis/organometallic homogeneous catalysis.
Journals (Please click-on the journal to access their websites)
Journal of the American Chemical Society
The Journal of Organic Chemistry
Advanced Synthesis & Catalysis
Chemistry – A European Journal
Asian Journal of Organic Chemistry
Rajesh Kunjanpillai completed his schooling from GHS Kizhakkupuram, Enathu and Jawahar Navodaya Vidyalaya (JNV), Pathanamthitta. He completed his undergraduate studies in Chemistry at St. Stephen’s College, Pathanapuram, in 2001. He obtained a B. Ed. Degree in Physical Science from MGUCTE, Pathanamthitta in 2003 after which he did his post-graduation in Chemical Sciences from the Department of Chemistry, Pondicherry Central University in 2005. He also cleared UGC CSIR (JRF-NET) and GATE. After his M. Sc., he got a selection as Research Associate in Shasun Research Centre (presently Strides Pharma Science Ltd), a Public Ltd pharmaceutical company in Chennai where he was actively engaged in drugs research, under the guidance of Dr. K. K. Balasubramanian, former Professor of IIT Madras. In 2006, he was promoted to Research & Development Officer at Shasun and garnered experience in pharma pilot plants. After more than 3 years of industrial experience, he joined as a Senior Research Fellow under Prof. Irishi N. N. Namboothiri, Department of Chemistry, IIT Bombay. In 2009, with a Ph.D. scholarship from the Swiss National Science Foundation (SNSF), he moved to the University of Zürich, Switzerland (Nobel Prize Laureates from the University of Zürich), where he was awarded his Doctoral Degree (D. Sc.) in late 2013, under the supervision of Prof. Heinz Berke (Prof. Heinz Berke). The title of his doctoral thesis was “Homogeneous Hydrogenations and Related Reductive Reactions Catalyzed by Rhenium Complexes.” He is also a recipient of the Swiss Chemical Society/Swiss Academy of Sciences Chemistry Travel Award 2012. In 2014, he joined as an Adjunct Faculty in IIRBS, M. G. University Kottayam, but later that year moved to IIT Bombay as an Institute Post-Doctoral Research Fellow.
Dr. Kunjanpillai was awarded the DST-SERB Govt. of India Young Scientist Fellowship and in July 2015, he joined as Assistant Professor in the Department of Chemistry, St. Albert’s College, Ernakulam. He has been serving as a visiting faculty, IIRBS, M. G. University since 2016. Dr. Kunjanpillai successfully completed a 3 year SERB research project and supervised a project assistant during that time. With the support from SERB and the Management of St. Albert’s College, Dr. Kunjanpillai set up a research laboratory with provisions for doing air and moisture sensitive reactions to a certain extent. He was recognized as a research guide by M. G. University, Kottayam in 2019. He is currently supervising three Ph.D. students. Dr. Kunjanpillai has more than 17 years of research experience and has published many research articles in reputed international journals. Notable work in his research include the nuclear bromination of deactivated aromatics using NBS. He has also developed a versatile strategy for the Claisen-Tishchenko reaction. Both the reactions are part of the UG and PG Chemistry Syllabus of universities/institutes in many parts of the world. His research interest includes organic synthesis, organometallics and homogeneous catalysis, activation of small molecules, developing atom economic and greener strategies for chemical synthesis etc.
During 2016-20, he had been serving as Secretary, Office of the Dean, Research and was the in-charge of the DST-FIST Facility of the College. Since 2020, he has been serving as the Dean, Research, & Academic Council member of the college.


Other Subjects of Interest
RK’s interest extends to gather theories and knowledge with regard to the origin and fate of the universe, evolution of life on earth, anthropology etc.
Interest is also keen in exploring the Vedic, Maurya, Sangam and the Vijayanagara literature, art and science.
Other than these, Carnatic music (both vocal and instrumental) is also a subject of interest.
Our broad areas of interest include organic synthesis, organometallics & catalysis.
Organic Synthesis: We work on developing greener and more efficient strategies/methodologies for organic transformations. Ligand sphere tuned transition metal/lanthanoid complexes are developed and applied as catalysts to carry out various reactions like aza-Michael/hydroaminations, transamidations, reduction of organic functional groups etc.
We are also interested in the total synthesis of natural products and pharmacologically active molecules.
Organometallics & catalysis: We also thrive towards synthesizing well defined organometallic complexes of non-noble metals aiming to apply for activating small molecules like H2, CO2, N2 etc. Interest is also keen in developing reductive catalytic processes for unsaturated organic substrates.

Ph.D. Students
Rajesh R. (2020 -; Pre-Ph.D. Presentation completed)
Topic: Co(II), Ni(II), Yb(III) and Tb(III) Catalyzed Michael-Type Hydroarylaminations of Activated Olefins

Meenu M. S. (CSIR-JRF; 2020 – 2022; CSIR-SRF – 2022 -)
Topic: Transition Metal Catalyzed Transamidation of Carboxamides and Ureas

Joseph Prince D. (Assistant Professor; 2022 -)
Topic: Greener Strategies for Aza-Michael Addition Reactions

Alex Kuriakose V. J. (Assistant Professor; 2023)
Topic: Reductive Amination of Aldehydes and Ketones using NaBH4 Catalyzed by Natural Citric Acids
PhDs Awarded

Rajesh R. (2024)
Title of the Thesis: Co(II), Ni(II), Yb(III) and Tb(III) Catalyzed Michael-Type Hydroarylaminations of Activated Olefins
Project Fellows

Rosmy Elizabeth (2022 -)
Project Students (Master Thesis)

Monica Beneeta Douravoo (2022-23)

Sanika Sabu (2022-23)

Anagha Titus (2022-23)

Ardra P. M. (2023-24; S. G. College, Kottarakkara)

Aswathy R. S. (2023-24; S. G. College, Kottarakkara)

Megha Raichel Shajan (2023-24; S. G. College, Kottarakkara)

Manjima K. (2023-24; MPMMSN College, Shoranur)

Shijitha (2023-24; MPMMSN College, Shoranur)

Arya O. S. (2023-24; MPMMSN College, Shoranur)

Manya Krishnan G. (2023-24; MPMMSN College, Shoranur)

Varsha Gopinath (2022-23; MPMMSN College, Shoranur)

Varsha E. (2022-23; MPMMSN College, Shoranur)

Shadiya A. P. (2022-23; MPMMSN College, Shoranur)

Amritha B. (2022-23; MPMMSN College, Shoranur)

Meghna (2022-23; St. Peter’s College, Kolenchery)
Past Project Fellows

Rajesh R. (Project Assistant; 2016-2019)
Past Project Students (Master Thesis)

Nihala Rasheed (2021; IIRBS, MG University, Kottayam)

Manu Jyothi Ravi (2021; IIRBS, MG University, Kottayam)

Jeny Jose (2021; IIRBS, MG University, Kottayam)

Nesrin V. A. (2021, St. Paul’s College, Kalamassery, Ernakulam)

Diya N. C. (2021; The Cochin College, Kochi)

Rekha Joseph (2021; The Cochin College, Kochi)

Sumayya P. M. (2021, The Cochin College, Kochi)

Arathi Karunan (2020; MPMMSN College, Shoranur)

Varna Stephan (2019-20)

Anju Vijayan (2019-20)

Sandhya O. K. (2019; MPMMSN College, Shoranur)

Nijina T. U. (2019; MPMMSN College, Shoranur)

Dolly David T. (2019; The Cochin College, Kochi)

Ruksana K. (2019; The Cochin College, Kochi)

Sella E. S. (2017-18)

Fenitta Albert K. (2016-17)
Research Articles
Lanthanoids in Hydroarylaminations: Yb(III) and Tb(III) Catalyzed Addition of Arylamines to Activated Olefins
Rajagopal Rajesh, Olencherry Karimpanakkal Sandhya, Sunilkumar Puthenpurackal Narayanan and Rajesh Kunjanpillai*
Abstract: Michael-type hydroamination of acrylonitrile, phenyl vinyl sulfone and dimethyl maleate were realized using arylamines catalyzed independently by Yb(OTf)3 and Tb(OTf)3 to give the desired β-amino acid derivatives or β-amino sulfones in moderate to excellent yields. The reactions were carried out in toluene for Yb(OTf)3 and in t-BuOMe for Tb(OTf)3, all carried out at 100 oC.
Synthesis, 2024
https://doi.org/10.1055/a-2282-7702
Thieme (Germany & United States)
A Simple and General Nickel Catalyzed Michael-Type Hydroamination of Activated Olefins Using Arylamines
Rajagopal Rajesh, Jai Anand Garg, Prabaharan Thiruvengetam, Rajesh Kunjanpillai*
Abstract: Nickel(II) catalyzed Michael-type hydroamination of activated olefins viz; acrylonitrile, phenyl vinyl sulfone and dimethyl maleate could be carried out systematically using 1–10 mol% of NiBr2 and 2–20 mol% of AgOTf. The reactions were conducted at 27–100 °C giving rise to the desired β-amino acid derivative or sulfone products in good yields.
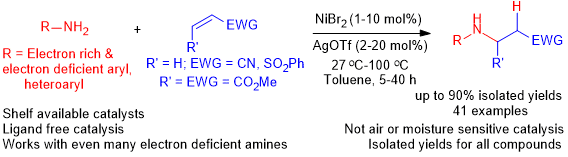
Asian Journal of Organic Chemistry, 2022 https://doi.org/10.1002/ajoc.202200440
Wiley-VCH GmbH, Weinheim, Germany
Crystal structure of a new 2,6-bis(imino)pyridine derivative: (1E,10E)-1,10-(pyridine-2,6-diyl)bis[N-(4-chlorophenyl)ethan-1-imine]
Rajagopal Rajesh, E. S. Sella, Olivier Blacque* and Rajesh Kunjanpillai*
The asymmetric unit of the title compound, C21H17Cl2N3, contains two crystallographically independent molecules (A and B). Both molecules have E configurations for both imine double bonds with regard to the aryl and pyridine groups. The conformations of the two molecules differ with the 4-chlorophenyl
rings being inclined to the central pyridine ring by 77.64 (6) and 86.18 (6) in molecule A, and 80.02 (5) and 43.41 (6) in molecule B. In the crystal, molecules are linked by a number of C—H interactions, forming layers parallel to the bc plane.
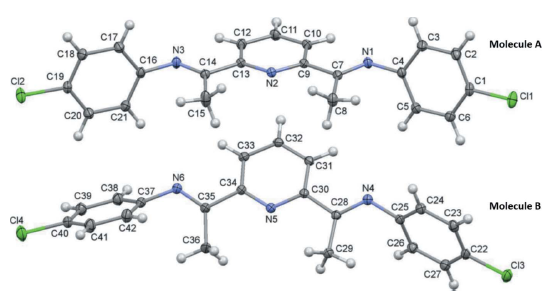
Acta Crystallographica Section E: Crystallographic Communications E75, 2019,115–118.
doi: 10.1107/S2056989018017966
International Union of Crystallography
Ullmann-Type and Related Redox Reactions of Nitrosyl Molybdenum Complexes Bearing a Large-Bite-Angle Diphosphine
Subrata Chakraborty, Rajesh Kunjanpillai, Olivier Blacque, Heinz Berke*
Abstract: The mild oxidation of [Mo0(NO)(P∩P)(NCMe)3][BArF4] P∩P = 2,2′-bis(diphenylphosphanyl)diphenyl ether, BArF4 = tetrakis[3,5-bis(trifluoromethyl)phenyl]borate with aryl halides affords dinuclear [MoI2(NO)2(P∩P)2(NCMe)2(μ-X)2][BArF4]2 species and biphenyl through an Ullmann-type dinuclear homocoupling process. The complexes are characterized spectroscopically and by X-ray diffraction studies.

European Journal of Inorganic Chemistry, 2016, 103-110. https://doi.org/10.1002/ejic.201501062
Wiley-VCH GmbH, Weinheim, Germany
Alkali Metal tert-Butoxides, Hydrides and Bis(trimethylsilyl)amides as Efficient Homogeneous Catalysts for Claisen–Tishchenko Reaction
Kunjanpillai Rajesh and Heinz Berke*
Abstract: Shelf-available alkali metal tert-butoxides, hydrides and bis(trimethylsilyl)amides were shown to be highly efficient homogeneous precatalysts for the disproportionation of aldehydes to the corresponding carboxylic esters. Potassium compounds in combination with 18-crown-6 ether could drastically increase the rate of reaction in a few cases. Alternatively, efficient aldol condensations were found for aldehydes possessing an enolizable methylene group at the α-position to the aldehyde functionality. The active species involved in this esterification using any of these alkali metal catalysts is expected to be the metal alkoxide. Potassium compounds were found to be much more efficient when compared to analogous sodium compounds and kinetic studies revealed the rate-determining step to be a second order concerted hydride transfer from a potassium hemiacetal species to another molecule of aldehyde.
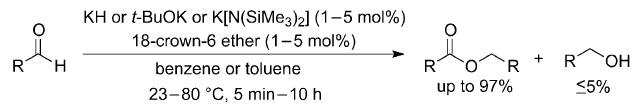
Advanced Synthesis & Catalysis, 2013, 355, 901-906. https://doi.org/10.1002/adsc.201200970
Wiley-VCH GmbH, Weinheim, Germany
Homogeneous Hydrogenations of Nitriles Catalyzed by Rhenium Complexes
Kunjanpillai Rajesh, Balz Dudle, Olivier Blacque, Heinz Berke*
Abstract: Rhenium(I) nitrosyl complexes bearing large bite angle diphosphines efficiently catalyze
the hydrogenation of nitriles to the corresponding symmetrical secondary amines.
Advanced Synthesis & Catalysis, 2011, 353, 1479-1484. https://doi.org/10.1002/adsc.201000867
Wiley-VCH GmbH, Weinheim, Germany
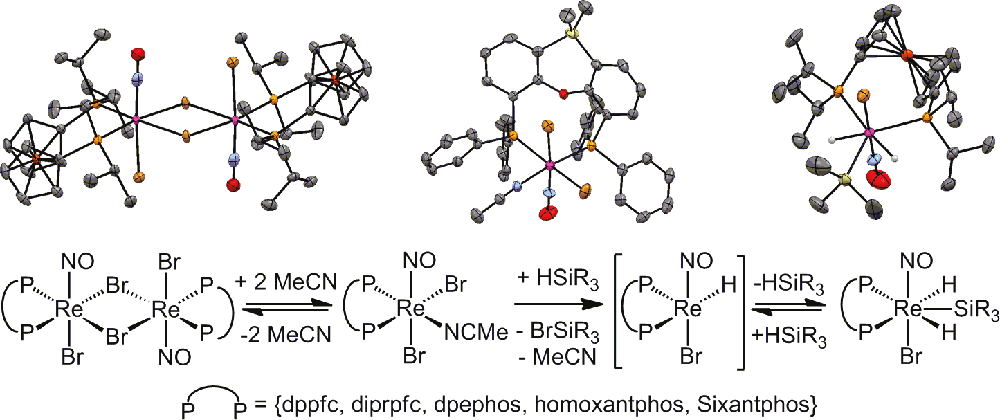
Rhenium in Homogeneous Catalysis: [ReBrH(NO)(labile ligand)(large-bite-angle diphosphine)] Complexes as Highly Active Catalysts in Olefin Hydrogenations
Balz Dudle, Kunjanpillai Rajesh, Olivier Blacque and Heinz Berke*
Abstract: The reaction of [ReBr2(MeCN)(NO)(P∩P)] (P∩P = 1,1′-bisdiphenylphosphinoferrocene (dppfc) (1a), 1,1′-bisdiisopropylphosphinopherrocene (diprpfc) (1b), 2,2′-bis(diphenylphosphino)diphenyl ether (dpephos) (1c), 10,11-dihydro-4,5-bis(diphenylphosphino)dibenzo[b,f]oxepine (homoxantphos) (1d) and 4,6-bis(diphenylphosphino)-10,10-dimethylphenoxasilin (Sixantphos) (1e)) led in the presence of HSiEt3 and ethylene to formation of the ethylene hydride complexes [ReBrH(η2-C2H4)(NO)(P∩P)] (3a,b,d), the MeCN ethyl complex [ReBr(Et)(MeCN)(NO)(dpephos)] (5c) and two ortho-metalated stereoisomers of [ ReBr(η2-C2H4)(NO)(η3–o-
ReBr(η2-C2H4)(NO)(η3–o- C6H4–Sixantphos)] 8e(up) and 8e(down). The complexes 3a,b,d, and 5c and the isomers of 8e showed high catalytic activity (TOFs ranging from 22 to 4870 h–1, TONs up to 24000) in the hydrogenation of monosubstituted olefins. For 8e(down) and 8e(up) a remarkable functional group tolerance and catalyst stability were noticed. Kinetic experiments revealed kobs to be first order in c(cat) and c(H2) and zeroth order in c(olefin). Mechanistic studies and DFT calculations suggest the catalysis to proceed along an Osborn-type catalytic cycle with olefin before H2 addition. The unsaturated key intermediates [ReBrH(NO)(P∩P)] (2a–e) could be intercepted with MeCN as [ReBrH(MeCN)(NO)(P∩P)] (10a–d) complexes or isolated as dimeric μ2-(H)2 complexes [{ReBr(μ2-H)(NO)(P∩P)}2] (9b and 9e). Variation of the bidentate ligand demonstrated a crucial influence of the (large)-bite-angle on the catalytic performance and reactivity of 3a,b,d, 5c, and 8e.
C6H4–Sixantphos)] 8e(up) and 8e(down). The complexes 3a,b,d, and 5c and the isomers of 8e showed high catalytic activity (TOFs ranging from 22 to 4870 h–1, TONs up to 24000) in the hydrogenation of monosubstituted olefins. For 8e(down) and 8e(up) a remarkable functional group tolerance and catalyst stability were noticed. Kinetic experiments revealed kobs to be first order in c(cat) and c(H2) and zeroth order in c(olefin). Mechanistic studies and DFT calculations suggest the catalysis to proceed along an Osborn-type catalytic cycle with olefin before H2 addition. The unsaturated key intermediates [ReBrH(NO)(P∩P)] (2a–e) could be intercepted with MeCN as [ReBrH(MeCN)(NO)(P∩P)] (10a–d) complexes or isolated as dimeric μ2-(H)2 complexes [{ReBr(μ2-H)(NO)(P∩P)}2] (9b and 9e). Variation of the bidentate ligand demonstrated a crucial influence of the (large)-bite-angle on the catalytic performance and reactivity of 3a,b,d, 5c, and 8e.

Journal of the American Chemical Society, 2011, 133, 8168–8178. https://doi.org/10.1021/ja107245k
American Chemical Society
Rhenium Nitrosyl Complexes Bearing Large-Bite-Angle Diphosphines
Balz Dudle, Kunjanpillai Rajesh, Olivier Blacque and Heinz Berke*
A series of [ReBr2(MeCN)(NO)(P∩P)] complexes (P∩P = 1,10-bis(diphenylphosphino)ferrocene (dppfc)(1a), 1,10-bis(diisopropylphosphino)ferrocene (diprpfc) (1b), 2,20-bis(diphenylphosphino)diphenyl ether (dpephos) (1c), 10,11-dihydro-4,5-bis(diphenylphosphino)dibenzo[b,f]oxepine (homoxantphos) (1d), 4,6-bis(diphenylphosphino)-10,10-dimethylphenoxasilin (Sixantphos) (1e)) were prepared with diphosphines varying in the P-Re-P bite angles. 1a,c–e were obtained from the reaction of [ReBr5(NO)][NEt4]2 with an
excess of the respective diphosphine in MeCN or MeCN/THF mixtures at elevated temperatures. Compound 1b was obtained by an alternative route, cleaving the dinuclear [{ReBr(μ2-Br)(NO)(diprpfc)}2] unit (2b) with MeCN. 2b was prepared from the reaction of [ReBr5(NO)][NEt4]2 with diprpfc in EtOH. The reaction of 1a–d with HSiEt3 gave the seven-coordinate [ReBr(H)2(SiEt3)(NO)(P∩P)] compounds 4a–d, of
which 4a,c,d are only stable in solution in the presence of HSiEt3. The SiMe3 (4f) and SiCl3 (4g) derivatives of 4b were also prepared by applying the reaction of 1b with HSiMe3 and HSiCl3. 1a,c,e, 2b, and 4f,g were structurally characterized. For 1c,e, 2b, and 4f,g NO/Br disorder was observed, which originates from the presence of two isomeric forms in the crystals of the respective compounds. For 1c,d fast interconversion of these isomers could be observed in their 31P{1H} NMR spectra at room temperature.
Organometallics, 2011, 30, 2986–2992. https://doi.org/10.1021/om200092y
American Chemical Society
One-Pot Three Component α-Aminoalkylation of Conjugated Nitroalkenes and Nitrodienes
Kunjanpillai Rajesh, Pramod Shanbhag, Manjoji Raghavendra, Pallavi Bhardwaj, Irishi N. N. Namboothiri*
Abstract: Nitroethylenes possessing aryl, heteroaryl, and alkyl substituents at the β-position as well as δ-substituted nitrobutadienes undergo facile aminoalkylation at the α-position upon treatment with formaldehyde and a secondary amine in the presence of imidazole and trifluoroacetic acid to afford α-aminoalkylated nitroalkenes and nitrodienes in good to excellent yield and stereoselectivity.
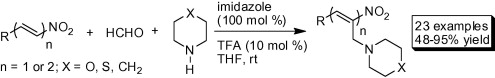
Tetrahedron Letters, 2010, 51, 846–849. https://doi.org/10.1016/j.tetlet.2009.12.015
Elsevier, Netherlands
Bromination of Deactivated Aromatics: A Simple and Efficient Method
K. Rajesh, M. Somasundaram, R. Saiganesh and K. K. Balasubramanian*
Abstract: Highly deactivated aromatic compounds were smoothly monobrominated by treatment with N-bromosuccinimide (NBS) in concentrated H2SO4 medium affording the corresponding bromo derivatives in good yields. Mild reaction conditions and simple workup provides a practical and commercially viable route for the synthesis of bromo compounds of deactivated aromatics.
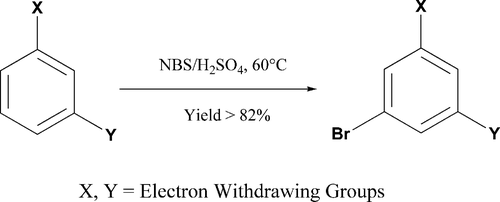
The Journal of Organic Chemistry, 2007, 72, 5867-5869. https://doi.org/10.1021/jo070477u
American Chemical Society
https://sites.google.com/ashoka.edu.in/suscat-sta-2024/speakers?authuser=0

https://sites.google.com/ashoka.edu.in/suscat-sta-2024/home?authuser=0
Metal Catalyzed Michael-type Hydroarylamination ofActivated Olefins and Transamidation Reactions
International Conference on Emerging Trends in Advanced Functional Materials (ETAFM-2022), 11 & 12 August 2022, Catholicate College, Pathanamthitta
Research and Publication Ethics in Science https://www.youtube.com/watch?v=GA0faeZXP8w&t=1625s
Office of the Dean, Training & Development, 27 July 2020, St. Albert’s College (Autonomous), Ernakulam
Nickel(II) Catalyzed Michael-Type Hydroamination of Activated Olefins using Arylamines
DST sponsored seminar on Green Approaches towards Chemical Synthesis, 07 & 08 November 2019, St. Gregorious College, Kottarakkara, Kollam
Metal Catalyzed Michael-Type Hydroarylamination of Activated Olefins and Transamidation Reactions
International Conference on Emerging Trends in Advanced Functional Materials, 11 & 12 August 2022, Catholicate College, Pathanamthitta
Hydrogenation of Carbon Dioxide to Methanol Catalyzed by Rhenium Diphosphine Nitrosyl Complexes
Albertian Knowledge Summit 2017, 26 January 2017, St. Albert’s College (Autonomous), Ernakulam
Hydrogenation of Carbon Dioxide to Methanol Using Large-Bite-Angle Diphosphine Nitrosyl Rhenium Catalysts
Annual Chemistry Symposium 2017, 23 September 2017, CMS College, Kottayam
Energy & Environment (General)
Chemistry Association Inauguration, July 2017, Maharaja’s College, Ernakulam
Chemistry & Sustainable Development (General)
Chemistry Association Inauguration 2022, St. Stephen’s College, Pathanapuram, Kollam
Funded Projects
Hydroamination and Hydroarylation of Olefins Catalyzed by Electrophilic Platinum Complexes
Agency: DST-SERB, Govt. of India (YSS/2014/000729)
Amount: INR 22,22,000/-
Duration: 2016-19

RK Research Group

With Prof. K. K. Balasubramanian, my mentor & well known organic chemist (Former Professor of IIT Madras) & Dr. M. Somasundaram (Director, Par Pharmaceuticals, Chennai) at the former’s residence in Chennai

With Prof. E. D. Jemmis at IISER TVM

With Prof. J. Gilliard (MIT) at IISER TVM

A word of thanks to Vidwan P. Ganesh (Chitraveena expert) at IISER TVM

An evening walk with Sri. Joseph Prince D. at IISER TVM

With Prof. J. N. Moorthy & Prof. K. George Thomas at Albert’s College, Ernakulam

# Congratulations Sri. Rajesh Rajagopal for successfully defending your Ph.D. thesis (17th April 2024).
# Paper published in Synthesis (Thieme, Germany & United States)
# Congratulations Ms. Meenu M. S. for being upgraded to CSIR-Senior Research Fellow w. e. from October 2022
# Congratulations Sri. Rajesh R. for being submitted the Ph.D. Thesis.
# Paper published in Asian Journal of Organic Chemistry (Wiley VCH-Weinheim, Germany)
Dr. Rajesh Kunjanpillai
Assistant Professor
Department of Chemistry
St. Albert’s College (Autonomous)
Ernakulam, Kerala – 682 018
India
E. mail: rajeshkunjanpillai@alberts.edu.in